Bcs Classification List
List for bcs classification - Download as Word Doc (.doc /.docx), PDF File (.pdf), Text File (.txt) or read online. Windows 7 ultimate 32 bit activation key generator free download. The BCS classification system is based on the scientific rationale that, if the highest dose of a drug candidate is readily soluble in the average fluid volume present in the stomach (250 ml) and the drug is more than >85% absorbed, then the in vitro drug product dissolution profiles should allow assessment of the equivalence of different drug.
Bcs Classification List Fda
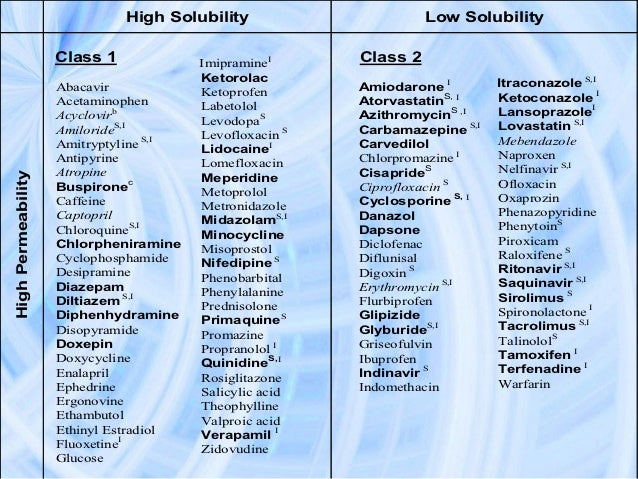

Bcs Classification List Of Drugs
The “” is an FDA guidance document, which allows pharmaceutical companies to forego clinical bioequivalence studies, if their drug product meets the specification detailed in the guidance. The principles of the BCS classification system can be applied to NDA and ANDA approvals as well as to scale-up and post approval changes in drug manufacturing. A waiver of In-vivo Bioavailability and Biioequivalence studies based on the BCS classification can therefore save pharmaceutical companies a significant amount of development time and reduce development costs. The BCS classification system is based on the scientific rationale that, if the highest dose of a drug candidate is readily soluble in the average fluid volume present in the stomach (250 ml) and the drug is more than >85% absorbed, then the in vitro drug product dissolution profiles should allow assessment of the equivalence of different drug formulations. Solubility and dissolution can be easily measured in vitro. 